Does Hydraulic Cement Continue to Harden With Water Exposure
Hydraulicity
Paul Livesey
| | ||
| The Eddystone lighthouse, now re-built on the Plymouth waterfront |
The term 'hydraulicity' is derived from the French word 'hydraulique' which, at its simplest is defined as relating to water. It was adopted into construction usage to describe waterproof structures either to convey water or to withstand the ingress of water.
In the early 18th century, French engineer Bernard Forest de Belidor used the term to describe construction techniques to resist the action of seawater. Early in the 19th century the eminent French engineer, Jean Louis Vicat, adapted the term to describe limes which would harden under water. They were thereby distinguished from the pure calcium limes, or 'air limes', which hardened by a different means resulting from the action of carbon dioxide when exposed to air. It was some time before the term came into common usage in Britain where an equivalent term 'water lime' had developed over a similar timescale. This initially arose with the work of Smeaton in developing his lime for seawater-resistant mortar for the Eddystone lighthouse (left) and continued through to the Portland cement precursor developed by Frost.
The term 'hydraulic' is now used internationally to describe cements and other binders which set and harden as a result of chemical reactions with water and continue to harden even if subsequently placed under water. There are a few exceptions but, in general, these chemical reactions involve calcium, silica and aluminium constituents which react with water to form a whole family of calcium silicate and calcium aluminate hydrates. Table 1 (below) sets out the chemical composition of typical hydraulic cements and binders.
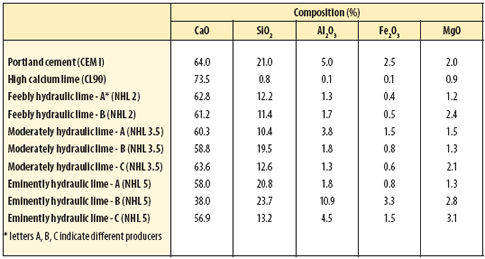 |
| Table 1 Main element oxide composition of hydraulic cements and limes |
The chemical composition in itself is only a limited guide to the hydraulic properties of the lime or cement. The key is in the way the components are combined into the various silicates and aluminates. Figure 1 (above) shows the relative strength obtained by the hydration of the common silicates and aluminates. In common with cement chemistry notation the four main compounds are described as:
- C3S - three parts calcium oxide combined with one part silicon oxide, also known as 'alite'
- C2S - two parts calcium oxide combined with one part silicon oxide, also known as 'belite'
- C3A - three parts calcium oxide combined with one part aluminium oxide
- C4AF - four parts calcium oxide combined with one part aluminium oxide and one part iron oxide, these latter two being generally termed 'aluminate phases'.
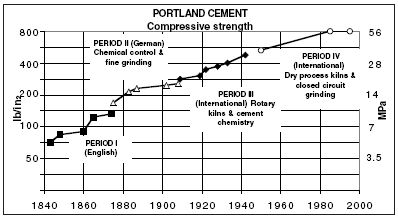 | |
| Figure 2 Changes in the properties of Portland cement (* MPa = megapascals: the unit of resistance to compression which is equal to one Newton per square millimetre) |
Although the overall bulk chemistry can be similar for different products, the burning process has a major bearing on their hydraulicity.
Limes are burnt at temperatures below 1,000°c where the main reaction products are belite and free lime together with small amounts of low reactivity aluminates. Portland cements are burnt at temperatures up to 1,500°c where almost all lime is combined, forming alite, which is the main product, and reactive aluminates. The development of hydraulicity as measured by compressive strength resulting from changes in technology over the past two centuries is illustrated in Figure 2 (right).
It can be seen that the calcium silicates are the main strength giving components in hydraulic limes and cements. The aluminate and ferrite compounds contribute little strength.
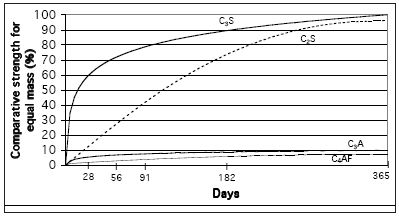 | |
| Figure 1 Comparative strength contribution of calcium silicates and aluminates |
POZZOLANS
Despite their lack of either a detailed knowledge of chemistry or the technology to burn at high temperatures, Greek and Roman engineers succeeded in producing 'hydraulic' constructions. They achieved this by combining a calcium-bearing constituent (that is to say lime) with products providing the silica and/or aluminium constituents. Such products are referred to as 'latent hydraulic' materials, that is: they are not hydraulic of themselves but become so when exposed to calcium-rich solutions. A number of materials fall into this category, both naturally occurring and manufactured. The best known traditional, naturally occurring example is the volcanic dust from the Mount Vesuvius region of Italy to the south of Naples. Termed pozzolana after the town of Pozzuoli around which the dust deposits are centred, the material was known by Roman builders to be capable of reacting with lime to produce superior mortars.
Other naturally 'pozzolanic' materials include the volcanic trass of the Rhine valley and various zeolites. Manufactured pozzolans are also commonly used today. These include metakaolin, a thermally treated china clay, and the waste by-products of several major industries such as blastfurnace slag, power-station fly ash and silica fume from the ferro-silicon industry. Thus the definition of a pozzolan could be a material that will react hydraulically with lime solution. Details of the composition of these pozzolans are set out in Table 2 (below).
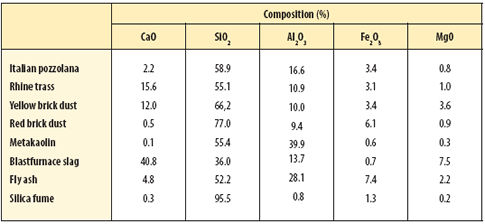 | |
| Table 2 Main element oxide composition of hydraulic pozzolans |
The degree to which such latent hydraulic materials react with lime is often termed their 'hydraulicity' or 'pozzolanicity' and a number of tests have been developed in order to measure this. The chemical test set out in BS EN 196-5 determines the quantity of lime absorbed from a standard lime solution by the pozzolan. In order to qualify for use in the standard pozzolanic cement of BS EN 197-1 a pozzolan must be capable of absorbing a specified amount of lime. Other more pragmatic tests have been developed to assess the strength-bearing properties in mortars and concretes. In the recent BS EN 206-1 concrete specification the term 'k factor' is used for latent hydraulic binders and is calculated as the amount of strength attributed to the pozzolan in concrete in comparison with a standard Portland cement. In the recent UK Foresight research into hydraulic limes a similar technique was used to determine the hydraulicity of pozzolans in mortar in comparison with an equal mass of the standard natural hydraulic lime used for the project.
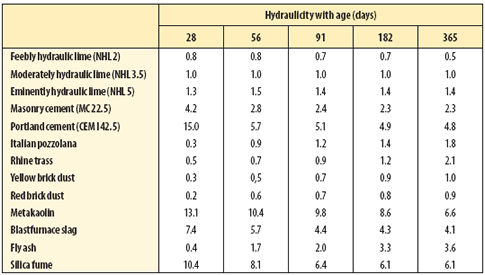 | |
| Table 3 Hydraulicity of cements and pozzolana compared with NHL 3.5 hydraulic lime |
Table 3 (above) sets out the hydraulicity of these pozzolans in relation to the natural hydraulic lime and cement. The pozzolans vary in their speed of reaction so that a measure of hydraulicity depends, to some extent, on the age at which it is determined. In this experiment small amounts of the pozzolans were added to a 1:3 moderately hydraulic lime mortar (1:3 NHL3.5:sand). The proportion of pozzolan to lime did not exceed 30 per cent by volume.
In addition to supplementing the strength of mortars and concretes, while utilising otherwise waste materials, latent hydraulic materials modify the hydration products formed and as a result modify the properties of the mortar or concrete. The values in Table 3 illustrate the various reaction rates of different silicates, different proportions of the silicates and decay of strength gain as either the reactive silicate or the available lime becomes depleted. The initial low hydraulicity of some pozzolans is ascribed to the slow diffusion mechanism whereby the reactive silicates are released for reaction with the lime-rich solution.
 | |
| Concrete caisson breakwater at Brighton Marina |
In summary, the term 'hydraulicity' is the property of limes and cements to set and harden under water whether derived from a naturally hydraulic lime, cement or a pozzolan. 'Cements' in this context can be single products or combinations of calcium bearing cement or lime mixed with materials that contribute reactive silica and alumina. The hydraulic characteristic of such materials is derived from their reactive calcium and silicon phases which combine with water to form calcium silicate hydrates of various densities. The density of the hydrate phases provides the binding power of the cement or lime and determines the strength of the mortar or concrete produced from it. The relative strength of mortar or concrete is used to quantify the 'hydraulicity' of the material. Products of moderate hydraulicity are capable of being used for mortar in exposed situations such as that shown in the title illustration. Products of high hydraulicity are required as the main construction material in extreme exposure situations such as that illustrated to the right.
~~~
Recommended Reading
- PC Hewlett (ed), Lea's Chemistry of Cement and Concrete, Arnold, London, 1998
- GC Bye, Portland Cement, Thomas Telford, London, 1999
- S Holmes and M Wingate, Building with Lime, Intermediate Technology Publications, London, 1997
This article is reproduced from The Building Conservation Directory, 2003
Author
PAUL LIVESEY, technical manager at Castle Cement Limited, is a chemist with 40 years' experience in the cement industry. He is lead UK expert to the British Standards and European Standards committees drafting the new generation of standards for cement, lime and methods of testing them, a director of Concrete Information Limited, Chairman of the British Cement Association Standards and Technical Committee and a member of the Building Limes Forum national committee.
Further information
RELATED ARTICLES
Lime Mortars and Renders
RELATED PRODUCTS AND SERVICES
Lime, hydraulic
Lime, hair & fibre reinforcements
Lime, non-hydraulic (lime putty)
Lime pointing
Lime, pointing tools
Lime, pozzolanic additives

Site Map
Source: https://www.buildingconservation.com/articles/hydraulicity/hydraulicity.htm

0 Response to "Does Hydraulic Cement Continue to Harden With Water Exposure"
Post a Comment